Age has a major impact on the cardiovascular system, affecting many aspects including cardiac rhythmicity, pumping ability, blood pressure and the vasculature. However, the effects of ageing are more than the result of a life-long exposure to detrimental factors such as hypertension, diabetes, cholesterol, smoking, and other risk factors. There is also an intrinsic physiology of ageing of the cardiovascular system, which is a slow and cumulative process, where only minute changes are observable over the short-term. These changes, however, accumulate over time, causing a progressive decline of cardiac function.
The Popeye domain containing (Popdc) genes encode a family of membrane proteins with high expression levels in cardiac muscle cells. Mutations in these genes in model organisms cause age-sensitive heart rhythm disorders, where the ageing-dependent traits resemble pathologies that are frequently observed in elderly patients. Research based at Imperial College in this area aims to provide a novel insight into the ageing process and potentially provide new therapeutic opportunities to help the ageing heart.
Cardiovascular disease is the leading cause of death and is responsible for about one third of all deaths. Population data such as that of the Framingham Heart Study demonstrate that the average incidence of the first cardiovascular event increases progressively with age, with 0.3% chance at the age of 40 and multiplying almost 25-fold to reach 7.4% at the age of 90.
The heart is the first developing organ that becomes functional in our body. Shortly after formation it starts to beat and will do so for the rest of our life. The average human heart rate is between 60-80 beats per minute, which translates into approximately 2.5 billion heartbeats over the course of a human life. The heart has four chambers that are tightly synchronized to pump blood at exact time intervals. The upper two heart chambers (right and left atria) help to fill the lower two larger chambers (right and left ventricle) with blood. The ventricles, in turn, drive the blood circulation throughout the body.
Cardiovascular disease is the leading cause of death and is responsible for about one third of all deaths
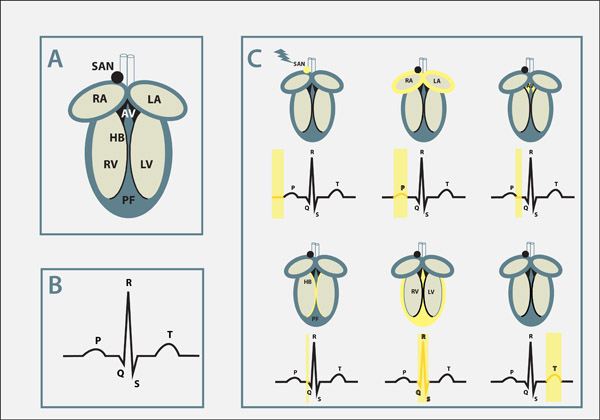
The heart has an electrical network of specialized muscle cells, the cardiac conduction system that synchronizes the contraction of the different heart chambers, but also controls the overall heart rate. A small cluster of cells in the right atrium, called the sinoatrial node, is the ‘pacemaker’, initiating electrical activation of the heart. Subsequently, electrical signals travel to the atrioventricular node (AV node), located between the atria and the ventricles, that acts as a ‘gatekeeper’ to control the speed of electrical conduction throughout the heart. Once electrical activity enters the ventricle, a group of specialized ‘electrical cables’ in the ventricle (the His-Purkinje network) is activated. Activation results in the rapid spread of electrical signals within the ventricles. The heart beats autonomously, but the autonomous nervous system, which stimulates the heart, has a modulatory role and is involved in speeding up or slowing down the beating rate.
Electrical activity travels in the form of an action potential (which can be understood as a melody) within heart muscles cells. What is also important to understand is that different parts of the heart have slightly different forms of action potentials. Thereby the melody played in these different muscle cells is similar but not identical. Such variations however are essential for a harmonious cooperation at the organ level assuring a proper heart rhythm from beat to beat.
Model Organisms for Ageing
Ageing induces structural changes in the heart. The number of cells that facilitate contraction (contractile cells) is reduced while the number of non-muscle cells is augmented. The accumulation of non-muscle cells increases the stiffness of the heart resulting in a lower ease of contraction and an impairment of the conduction of electrical signals. Thus, cardiac arrhythmia (i.e. atrial fibrillation, ventricular arrhythmia or insufficient activity of the cardiac pacemaker), are prevalent conditions in the elderly that may cause sudden cardiac death if left untreated.
Ageing can be studied in model systems with a short lifespan since they have a relatively accelerated ageing process (e.g. small rodents and flies). Diastolic dysfunction is an age-related condition where there is an impairment of relaxation of the heart at the end of each contraction cycle causing a reduction in the net transport of blood. The underlying mechanisms of diastolic dysfunction may include an increase in the thickness of the ventricular wall (myocardial hypertrophy), changes in the ratio of non-muscle to contractile cells within the muscle tissue of the heart (myocardium), and changes in gene expression.
Ageing induces structural changes in the heart. The number of cells that facilitate contraction (contractile cells) is reduced while the number of non-muscle cells is augmented
Significantly these changes can also be observed in aging mice and rats. At baseline the heart rate of a mouse amounts to approximately 450 beats per minute and in response to physical activity the beating rate increases to about 700 beats per minute. These beating rates are significantly different from that of a human heart, which beats at rest between 70-80 beats per minute and physical activity causes an increase to up to 200 beats per minute. Such vast differences in the beating rate cast some doubt on the suitability of the mouse as a model to study the basis of cardiac arrhythmia. However research in the last 15 years has demonstrated that there is sufficient similarity between both mammalian species to study the role of genes as the molecular basis of heart rhythm control in mice.
The fruit fly Drosophila, which has a life span of only a few weeks, has also been successfully utilized to study the process of ageing. As in humans, Drosophila’s heart function declines with age and the incidence of many heart conditions increases significantly with the age of the fly. Genetic analysis revealed several pathways that may be responsible for these age-related changes. However, not all pathways are identical between species and significant further research is required to apply the knowledge gained in model organisms to patients.
The Popeye Domain
When we are running or cycling, the beating rate of our heart starts to ramp up in order to increase blood perfusion of our organs and to meet the increasing oxygen and nutrition demands. This so-called fight-or flight response is essential for increasing cardiac output under stress. Elderly people often have an impaired response to physical activity. They frequently suffer from sick sinus syndrome. With this disease the heart rate is often normal at rest. However when affected patients have to perform physical activity, for example, when they are climbing stairs, the impaired ability of the pacemaker to increase the heart rate causes dizziness or even unconsciousness. Therapeutic intervention of sick sinus syndrome involves the implantation of an electrical pacemaker device. Sick sinus syndrome is reasonably prevalent and occurs in about 1/600 cardiac patients aged 65 years or older. About 50% of all pacemaker implantations are due to the diagnosis of sick sinus syndrome, with about 26,000 in the UK alone at a cost of £43 million per year.
About 50% of all pacemaker implantations are due to the diagnosis of sick sinus syndrome, with about 26,000 in the UK alone at a cost of £43 million per year
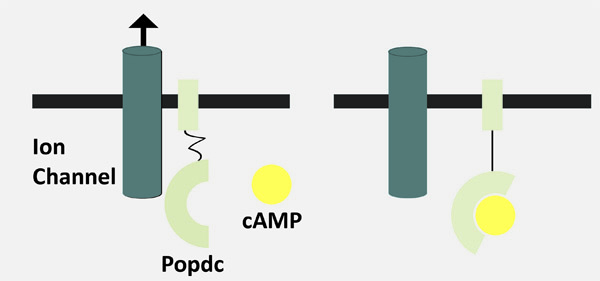
In this context the Popeye domain containing (Popdc) gene family was discovered in the search for genes with a strong and specific expression in the developing heart. Intriguingly, despite the strong expression in the developing and adult heart, the experimental inactivation of Popdc genes in mice had no apparent effect on the developing heart.
Popdc genes are present at high levels in the cardiac conduction system (See schematic representation), but under resting conditions Popdc mutant mice display no sign of a cardiac arrhythmia. However, the mutant animals revealed their disease phenotype only when subjected to stress.
In Popdc mutants a specific form of cardiac arrhythmia, an abnormally slow heartbeat, (bradycardia) was observed when the animals were subjected to physical activity such as running or swimming. Instead of the 700 beats per minute, seen under stress in normal mice, the mutant animals had a heart rate approximately 100 beats lower. This reduction in the maximal heart rate was due to a specific defect in the sinoatrial node (the heart’s pacemaker tissue). Instead of rhythmic pacing of the heart, the mutant sinoatrial node displayed intermittent pauses, which occurred randomly and were of different length in time. This can have a dramatic impact since the heart does not perform according to the physiological demands of the body.
Another important aspect, which makes the Popdc mutant animals very valuable models, is the fact that the bradycardia is not present in mutant mice at a young age. It only becomes apparent when the animals get older. The Popdc mouse mutants are therefore excellent models to study the molecular processes of ageing, which lead to sick sinus syndrome. These animal models may also help to develop novel therapeutic interventions for this disease. The pathological phenotype in the mouse mutants resembles to a large extent the human condition. The stress dependence and the random occurrence of pauses are exactly what have been observed in patients with this condition. Knowing the time-point at which the bradycardia phenotype is first present, one can determine what has changed at the level of the organ, cell or even gene expression. This will allow for the specification of the molecular mechanism through which age has an impact on heart function.
An important question that also needs to be addressed is whether mutations in Popdc genes in patients are associated with cardiac arrhythmia. Moreover, will a bradycardia phenotype be always present in patients with a mutation in Popdc genes? An answer to this question may come from the search for patients with cardiac arrhythmia that have mutations in Popdc genes. This search is currently being pursued through collaborations between Imperial College and various clinics in the UK and in Europe.
It is worthwhile to discuss in this context another set of experimental data that has been obtained in zebrafish. The zebrafish has recently become a prominent model organism, which is now widely used in biomedical research. What makes this animal model so attractive for heart research is the fact that the fish heart beats at a very similar rate to the human heart. Moreover, the zebrafish is transparent and the embryonic heart can be easily monitored with the help of a microscope. Strikingly, the fish heart utilizes a similar set of ion channels to humans and the phenotypes of several genetic mutations are more comparable with humans in the fish than in the mouse. Cardiac arrhythmia is also present in zebrafish lacking Popdc gene function. In contrast to the mouse however, the phenotype is associated with a deficiency of the atrioventricular node (AV), which acts as a gatekeeper to regulate electrical activation of the ventricles. Thus, at present it is unclear what kind of cardiac arrhythmia will be observed in patients with mutations in Popdc genes. Will it be more similar to the zebrafish or the mouse phenotypes? Nonetheless, given that cardiac arrhythmia phenotypes are observed in two model organisms, which are only very distantly related, it is quite likely that mutations in Popdc genes will have an impact on the electrical activation pattern of the human heart.
Studying the role of the Popeye domain containing genes in the heart will help to better understand cardiac arrhythmia and the ageing heart. However, one needs to be cautious about extrapolating results from animal models to humans. This is particularly true for ageing research. A prominent example is caloric restriction. In roundworms, yeast, and fruit flies caloric restriction significantly extended life span, but a recent study in monkeys did not provide any evidence for a life-extending effect of caloric restriction. It is currently thought that life expectation in man may be mostly influenced by genetic networks and not so much by environmental factors such as diet or lifestyle. It is thus probably very difficult to fully model human ageing in any model organism. Nonetheless ageing research in model organisms will provide us with concepts and biological targets, some of which may indeed have an impact on the ageing process in humans. It may not increase our longevity but possibly improve our health and the quality of life during ageing.








